Gut bacteria are essential to human health and recent research is showing how they work
Published on 10 March 2011 in Food, health and wellbeing
Introduction
We are not alone. Each and every one of us plays host to millions of bacteria (bugs), the majority of which reside in our guts. These microscopic organisms are mostly friendly bugs and are essential to maintaining human health.
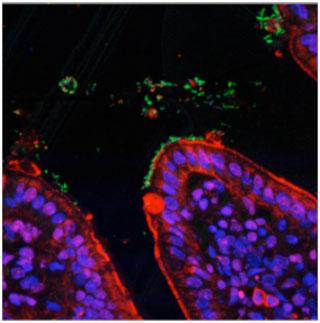
Over the last decade there has been a global increase in scientific research in the areas of human gut microbiology and immunology with millions of US dollars, Euros, Yen and UK pounds invested in progressing and advancing these scientific areas. Such a large financial spend is totally justified when we consider the huge number of human diseases including infections, cancers, inflammatory and autoimmune diseases, as well as allergies, which are caused by the failure of the human immune system to function properly. Microbes are singly the most important factor driving immune development, education and function and it is the combined effort of microbiologists and immunologists that is advancing our understanding of how microbes and immunity contribute to human-health and well-being. Image shown: Bugs (Green) and Guts (Red/Blue)
Bugs influence the human immune system in very diverse and profound ways. The evidence demonstrating that our natural living environments, or more specifically microbial biodiversity within our living environments, can influence microbial colonisation of the gut as well as the functioning of the human immune system is compelling.
Key Points
The dramatic increase in the incidence and prevalence of chronic inflammatory diseases in Westernised countries and the heightened allergy risk associated with urban (not rural) life styles are now recognised to be linked to variations in microbial diversity within these environments.
Current research on human gut bacteria is often packaged and presented under the umbrella term ‘Probiotics’. However, probiotics is not a new concept. Traditionally, probiotics have been linked with numerous human health benefits most of which have been driven by billion dollar marketing campaigns and based on limited scientific evidence.
Net result: the scientific literature is awash with contradictory reports regarding the efficacy and actions of these probiotics and the failed attempts to obtain European Food Safety Authority (EFSA) endorsement and regulatory approval, further fuels the notion that probiotics are products of hype and no substance.
Probiotic research in its broadest sense is now mistakenly regarded as mature science. The implication being that we have discovered all there is to know about probiotics, bugs and human health. This is emphatically not the case.
The challenge now, and for the future, is to ensure that the scientific breakthroughs and advances in our understanding of how gut bugs positively influence the human immune system and human health are successfully harnessed and translated into tangible health benefits.
Research Undertaken
.jpg) Our research recently published in BMC Biology, (also reviewed in Nature), clearly supports the important contribution of environmental microbial diversity to microbial colonisation of the gut and immune development in early life. Image shown: Bacteroides theta ‘A better choice for Probiotic’ (A D Phillips).
Our research recently published in BMC Biology, (also reviewed in Nature), clearly supports the important contribution of environmental microbial diversity to microbial colonisation of the gut and immune development in early life. Image shown: Bacteroides theta ‘A better choice for Probiotic’ (A D Phillips).
We, along with others, have now demonstrated that all bugs are not equal. Our work, published in the scientific journal ‘Immunity’, contributes to the growing evidence that individual bacterial species, and indeed different strains of the same bacterium, influence immune development and function in very distinct ways.
We continue to define how bugs alter the human immune system. We have found that certain gut bacteria promote cells which drive active immune responses in the gut (effector cells), whereas other bacteria, promote cells which maintain immune homeostasis (regulatory cells). The balance of these effector and regulatory cells is critical to gut and systemic health.
We can now identify highly evolved gut bugs, or their constituent parts, and use them to prevent or treat human diseases associated with a dysfunctional immune system, such as inflammatory bowel disease. For futher information visit the GT Biologics website.
Policy Implications
By understanding the biology of gut bugs and the way in which they regulate the human immune system, we can identify those bugs whose physiological functions are fit for purpose. This work will drive innovation, product development and policy advice and, by design, will meet the standards expected by EFSA experts. So the real challenge in the future is to ignore the negative probiotic hype and let the current innovative science speak for itself!
Author
Professor Denise Kelly d.kelly@abdn.ac.uk





