Folate And Cancer
Published on 19 March 2009 in Food, health and wellbeing
Introduction
Folates, a family of water-soluble B vitamins play a crucial role in the development of human diseases such as heart disease in adults, cognitive dysfunction and dementia in the elderly and congenital defects in babies. Low folate status has also been implicated in cancer development. Folate deficiency is widespread. Compelling evidence for a protective effect of folic acid supplementation for prevention of neural tube defects (NTDs) in the newborn prompted the UK Government to consider introducing mandatory fortification of flour with synthetic folic acid. This research describes how increasing or decreasing folate in cells in culture, in animal experiments and in human studies alters DNA stability. We have shown that severe folate deficiency acts to destabilise DNA and increase risk of malignant transformation. We have also found that increasing folate status (either in cell experiments or in human volunteer studies) can improve DNA stability.
Key Points
Folate deficiency is widespread, with 40% of 15-18 year olds in the UK exhibiting marginal folate status and folate deficiency common in people over 65 years of age, especially in the institutionalised elderly.
Folate deficiency has also been implicated in the development of cancer, notably of the cervix, lung, breast, brain and colon. The evidence linking folate deficiency and human carcinogenesis is strongest for the colon. However, new data suggest that increasing folate intake to pharmacological levels in both animals and in humans, may actually accelerate carcinogenesis.
In order to establish how increasing folate status modifies cancer risk (both positively and negatively) in humans, it is essential to understand mechanistically how folic acid alters DNA stability at the molecular level.
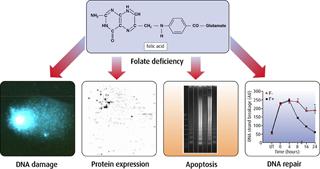
Folates are crucial in the synthesis of DNA and are also key regulators of cellular gene and protein expression. Any perturbations in these processes, due to an inadequate folate supply from the diet, are likely to severely disrupt the cells ability to replicate and divide properly and may increase the likelihood of that cell becoming cancerous. Folate deficiency may decrease DNA stability by inducing uracil misincorporation into DNA (which causes DNA strand breakage), by altering DNA repair and by inducing abnormal DNA synthesis.
Our research has demonstrated that-
• folate deficiency in normal cultured human colon cells (colonocytes) causes breaks in DNA.
• DNA strand breakage in human lymphocytes and colonocytes can be prevented by increasing folate levels to concentrations found in normal human blood.
• the ability of colonocytes to repair their DNA is inhibited by folate deficiency.
• folate deficiency alters DNA synthesis and prevents the colon cell from dividing normally.
• several biochemical pathways implicated in carcinogenesis are altered by folate deficiency in human colon cells.
• folate deficiency in rats also causes DNA breakage and alters DNA repair activity in the liver and colon.
• folate supplementation in human volunteers with a habitually low dietary intake of folic acid actually improves the stability of the DNA molecule.
Research Undertaken
The effect of altering folate status on genomic stability has been investigated both in model systems and human studies. We have found that DNA strand breakage and apoptosis occurs in folate-deficient cultured human colon mucosal cells. Moreover, DNA base excision repair in response to oxidative stress is significantly impaired in these cells and is associated with abnormal DNA synthesis. This effect is concentration-dependent at levels found in normal plasma (1-10ng/ml), indicating that folate levels that prevent overt deficiency may not be optimal in maintaining DNA stability.
Recently, using a novel proteomics approach to measuring total protein expression in human colon cells, we have identified proteins and pathways implicated in malignant transformation that are significantly altered by folate status. Folate deficiency causes uracil misincorporation in rat lymphocytes and a decrease in repair of oxidative and alkylation lesions in the DNA molecule, two types of DNA damage implicated in human cancers. Conversely, increasing folate intake actually improves DNA stability in people with low, but normal blood folate levels. Human volunteers were given 1.2mg folate for 12 weeks and DNA stability and incision repair activity were measured in their lymphocytes. Folate supplementation increased blood folate concentrations and decreased uracil misincorporation in these subjects.
Policy Implications
Compelling evidence for a protective effect of folic acid supplementation before conception in preventing neural tube defects (NTDs) in the newborn prompted the UK Government to consider introducing mandatory fortification of flour with synthetic folic acid. The Scientific Advisory Committee on Nutrition (SACN) evaluated the evidence for the benefits and disadvantages of compulsory fortification and published its findings in 2006 [Scientific Advisory Committee on Nutrition (2006), Folate and Disease Prevention, London, The Stationery Office]. The decision whether to fortify will probably be made by the UK Government later this year.
This research describes how increasing or decreasing folate in cells in culture, in animal experiments and in human studies alters DNA stability. We have shown that severe folate deficiency acts to destabilise DNA and increase risk of malignant transformation. We have also found that increasing folate status (either in cell experiments or in human volunteer studies) can improve DNA stability.
With regards to public health and policy making, these data suggest that increasing folate intake in humans with a low habitual folate intake would be beneficial in terms of maintaining genomic stability. However, it remains to be established whether increasing folic acid intake significantly above that normally consumed in the diet has any long-term detrimental effects. Animal data describing increased malignant transformation in rats in response to high folate and recent reports of increased risk of human cancers (breast and colon) in people exposed to high folate, either through food fortification or supplement use, suggest that persistently high circulating levels of unmetabolised folic acid may cause problems for susceptible groups such as people undergoing chemotherapy or individuals with undiagnosed cancer.
We are currently investigating how high folate alters genomic stability in experimental systems.
Related documents:
Duthie SJ et al., J. Proteome Res., (2008), 7: 3254-3266.
Duthie et al., Proc. Nutr., Soc., (2007), 65: 105.
Basten et al., Brit. J. Cancer (2006), 94: 1942-1947.
Duthie SJ et al., Proc. Nutr. Soc., (2005), 63: 571-578.
Duthie SJ et al., Nutrition & Cancer (2000), 37, 127-133.
Author
Dr. Susan Duthie S.Duthie@abdn.ac.uk







